સોડિયમ બાયકાર્બોનેટ
ઉત્પાદન વિગતો:
- ઉત્પાદન પ્રકાર
- ફોર્મ
- સંગ્રહ સૂચનાઓ OTHER
- વધુ જોવા માટે ક્લિક કરો
સોડિયમ બાયકાર્બોનેટ ભાવ અને જથ્થો
- 1000
- કિલોગ્રામ/કિલોગ્રામ
સોડિયમ બાયકાર્બોનેટ ઉત્પાદન વિશિષ્ટતાઓ
- OTHER
સોડિયમ બાયકાર્બોનેટ વેપાર માહિતી
- bhavnagar
- સપ્તાહ દીઠ
- અઠવાડિયું
ઉત્પાદન વર્ણન
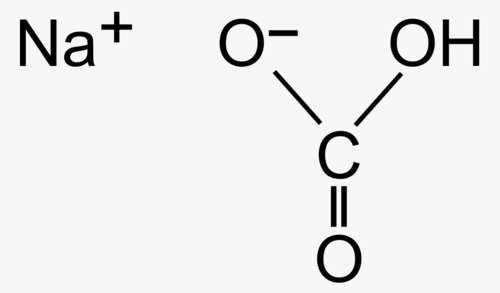
Sodium bicarbonate commonly known as baking soda is a chemical compound with the formula NaHCO It appears as a white crystalline powder or a fine granular substance Here are some key details about it
Chemical Properties
Molecular Weight 8401 gmol
Appearance White powder
Solubility Soluble in water
pH Typically around 84 in a solution
Uses
Baking As a leavening agent it helps dough rise by releasing carbon dioxide when combined with an acid and moisture
Cleaning Its mild abrasiveness and ability to neutralize odors make it a popular ingredient in household cleaning products
Medical It can be used to treat acid indigestion or as an antacid and it also plays a role in certain medical treatments such as kidney dialysis
Deodorizing It can neutralize odors in refrigerators carpets and other areas
Fire Extinguishing Used in some types of fire extinguishers particularly for grease fires
Safety and Handling
Generally regarded as safe for most uses but inhaling large amounts of dust or prolonged contact with eyes or skin should be avoided
In high doses it can cause a condition known as metabolic alkalosis where the blood becomes too alkaline
Storage
Should be kept in a dry place in a tightly sealed container to prevent moisture absorption and clumping
Overall sodium bicarbonate is a versatile substance with a wide range of practical applications in both household and industrial settings

Price: Â
- 50
- 100
- 200
- 250
- 500
- 1000+

 Get a Quote
Get a Quote